
Atoms
Atoms are tiny spheres that all materials are made of. There are over 100 different kinds of atoms, as seen in the table below (called the "Periodic Table", which groups similar atoms together in the same column). Atoms are made of even tinier pieces: protons (p+) in the center, usually joined by neutrons (n0), and circling around the outside are electrons (e-); more details of the table here.
*CLICK ONCE ON TABLE TO ENLARGE
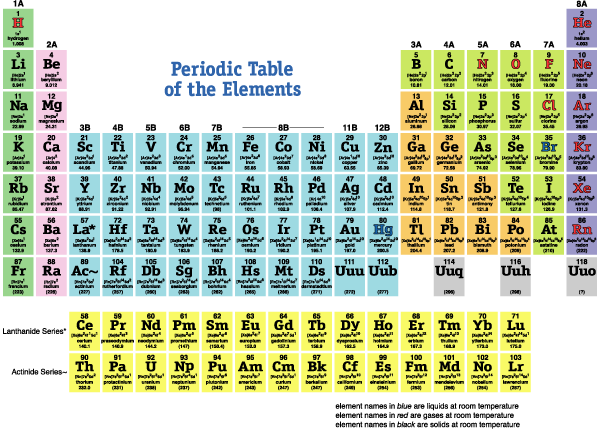
Some basic patterns in this table:
- the simplest atom H (1 p+ and 1 e-) is listed at the top left
- atoms get "bigger" (more +'s in their center) from left to right, and top to bottom
- atoms in the same column have similar chemical properties (e.g. the same number of
chemical bonds)
- atoms more to the right hold e-s more tightly, except the rightmost
- atoms listed in the rightmost column usually don't combine at all to form molecules
|
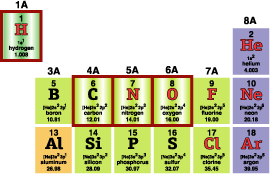
A very large part of estuary science focusses on just a few atoms from this collection, namely:
- H, hydrogen, the smallest, lightest,
simplest atom
- C, carbon, top of column IV A
- N, nitrogen and
- O, oxygen, follow C in the list
- Na (sodium), Cl (chlorine), and maybe
Ca (calcium)—involved in salts in ocean water
|

|


|
More:

Modelling Chemical Bonds
Q: What's the simplest atom?
A: H, hydrogen
Q: What's the simplest molecule (combination of atoms)?
A: H2, just 2 of the simplest atoms sticking to each other.
Make a model by taking a (1/2-length) toothpick and using it to attach 2 small blue styrofoam balls together. According to the Periodic Table, H forms just one bond (represented by the single toothpick).
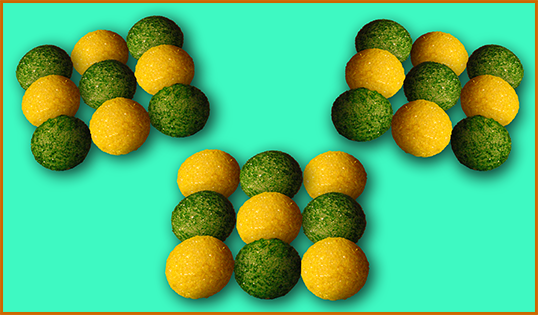
Now for a slight complication: O can form 2 bonds, so how do 2 O's join together? Like 2 people holding each other with both hands, each O holds the other with both bonds. In the pictures below, each pipecleaner is one bond; 2 bonds between the same 2 atoms is a "double bond", and pipecleaners are good for representing them because they can bend.
    
Try a hands-on activity to practice these ideas and see them in action!
A slightly more complicated example: C has 4 bonds, O has 2; so one C can double bond to 2 O's (above/right), making CO2, or "carbon dioxide" ("di" means "2", for the 2 O's in the molecule).
Note that these 3 molecules are straight lines. Other molecules, even H2O, are more complicated...


|
More:

Modelling Salt (NaCl)
Salt is an essential ingredient of an estuary. Basic science ideas about salts (especially NaCl) can effectively be explored by hands-on modelling of crystals of NaCl (table salt). These activities use multiple senses to encode information, and are fun, and conceptually simple.
Forming a crystal
Imagine a solution of NaCl, as the H2O evaporates. With less and less water present, the Na+ and Cl- ions come closer and closer together. Opposites attract, likes repel, so which of the following will (will not) happen?
  
Either of these possible?
  
Either of these possible?
As the (possible) trios above crowd together, which of the following will happen?
 OR OR  OR OR  ? ?
More water evaporates, more Na's and Cl's stick together:
 |
Yet even more water leaves, and these larger clumps stick to each other...

producing crystals of characteristic shape (as seen
through a microscope).
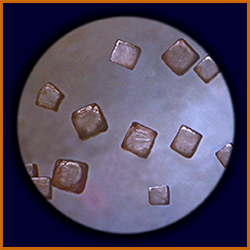
|
|
More:

More Complicated Molecules
In the Periodic Table, the positions of the columns indicate how many atomic bonds
can usually be formed, as follows: |
- For C, N, O (outlined in red, to the right—these are the most important atoms for life):
- C forms four bonds, (same for Si, Ge,...)
- N, P, As,...(usually) form three (NH4+ is an interesting
exception),
- O, S, Se,...form two
- H forms one (as seen above),
- He, Ne, Ar, Kr in the rightmost column form 0.
|

|
This rule is shown by the combinations of H with C, N, O: |
The Shapes of Molecules
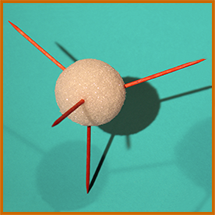
To make the shapes of the molecules named above as realistic as possible, the angles between the H's should be right. Methane's H's are at the 4 points of a tetrahedron, and ammonia and water are very similar (but with some vertices not having H's).
You can print out a cardboard guide like the one on the right, showing where to stick the toothpicks into the styrofoam spheres by folding a cardboard tetrahedron of just the correct size (2 x 61/2 times the diameter of the sphere) around the sphere,...then skewer it through the centers of the bottom face and 1, 2, or all 3 of the other faces, resulting in something like the photos below. You may need to print the guide larger or smaller, depending on the size of your styrofoam sphere. (Note the slit for folding the face away from the sphere without having to remove the toothpick.)
For Oxygen, only 2 toothpicks would be used, for Nitrogen, 3; if double bonds are planned, pull out the toothpicks involved and replace with pipecleaner segments.
|
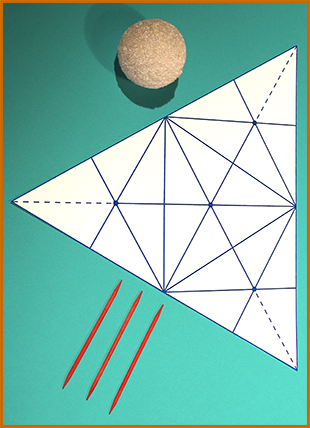

|
 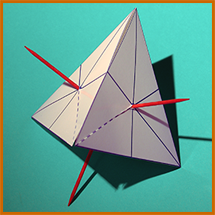  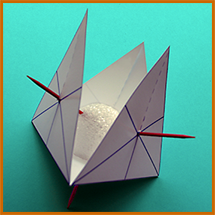  


|
More:

Energy and Heat
Energy is, in a way, the "money" of nature—nothing can happen without it, it comes in many forms that can be changed into each other without changing the amount (like a dollar bill changed for 4 quarters or 10 dimes or any other combination). Living things must get it from somewhere, and spend it to do the things they need to do. It's NOT made of atoms (so it's not a material) but atoms and molecules can have energy in them. It can also be present without any atoms nearby—e.g. light in outer space.
Some types of energy are:
- motion—any material object, small or large, from pieces of atoms to galaxies, has energy if it's moving, more energy if it's faster (E = mv2/2)
- atoms and molecules are constantly moving and jiggling; this energy is called "heat", and faster motion is more energy is hotter
- light is a kind of energy that doesn't need atoms; when it hits atoms it can make them move (light energy converted into heat—does Sunlight feel warm?)
- chemical energy is stored in fuels and batteries
- electrical energy can be converted into light (lightbulb), heat (electric stove), motion (electric motor), etc.
- many other kinds...
Heat Energy
Heat is a particularly important form of energy—it's everywhere, it has many important effects (in particular, on the estuarine environment), and its various effects are easy to understand. A good exercise in "Scientific Method" is to make predictions based on the "kinetic theory of heat" i.e. heat is the motion of atoms and molecules", then test those predictions experimentally.
Pondering some questions might suggest suitable predictions, and ways of testing them:
- Which takes up more room, cold or hot water? Can you name a practical invention that exhibits the answer?
- Which will break apart and dissolve a salt crystal faster, cold or hot water? Why? Try it experimentally!
- What happens to a solid whose molecules move faster and faster? (It melts—crystal structure breaks apart.)
- What happens to a liquid whose molecules move faster and faster? (It boils—molecules separate and fly about independently, singly.)
- What happens to a gas whose molecules are slowed down? (It condenses.)
- Will a gas dissolve better in hot or cold water? (Cold water slows down and captures gas molecules better—very important question for life in estuary waters!)


|
More:

Burning Fuels
Fuels are chemicals with energy stored in them, that can be released by burning.
And what is "burning", anyway? Fires need fuel and oxygen; the process of burning is the molecules of oxygen and the molecules of fuel breaking each other apart and reforming into new combinations.
- Coal is made of carbon atoms; the burning of carbon involves carbon atoms attaching to oxygen atoms.
- In the air, oxygen (O) atoms like to stick together in pairs—so written as O2.
- If there's enough air, the result is carbon dioxide CO2; if there's a shortage of air, carbon monoxide CO can be formed.
|

C (carbon)

O2 (diatomic oxygen)

CO2 carbon dioxide—same atoms as above, now joined all together |
       
Hydrogen gas (H2) is also a fuel that can burn with oxygen; 2 molecules of hydrogen
combine with one molecule of oxygen to form?
As a chemical formula: 2H2 + O2 -> 2 H2O + heat and light (if combination occurs via fire). A fuel cell is an apparatus for combining them and producing electricity instead of light and heat—and NO POLLUTION! Just water as the exhaust! |
Another fuel that's very important for us is natural gas,
used in:
- stoves
- water heaters
- furnaces
- electric power stations
and many other places. Chemists call it methane, and its molecule contains 4 hydrogens (H) stuck to one carbon (C)—so written as CH4. |

CH4 (Methane)

O2 (Oxygen) |
Here's a hands-on activity to work with these ideas in the classroom!


|
More:

Dissolving
A reverse process of crystallization is dissolving, whereby a solid like salt is put in water and magically disappears! How does this happen? Students who like puzzles and magic tricks can be motivated to figure out this magic trick of Nature, with hands-on activities to help then visualize and understand.
|
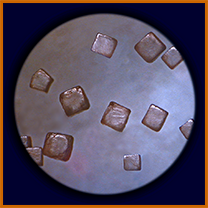 |
A salt crystal in water is continually pounded by moving water molecules, which can chip off individual atoms of Na or Cl (too small to see) and carry them away. If there's enough water, the whole crystal is eventually broken up completely and spread through the water, becoming invisible. Questions to ponder:
- The molecules of hot water move faster, so will hot water be more effective at dissolving salt, or less effective? (Prediction #1)
- For cubic crystals like those shown above, what parts will break off earliest? (Prediction #2)
Design experiments that test these predictions.
The atoms of Na are + and stick to the O's of H2O, which are -; the Cl's are - and stick to the H's of H2O, which are + (this is how the salt's atoms get carried off). Here's another question to ponder: pure water is a very poor conductor of electricity; does adding Na + s and Cl-s change this? (Prediction #3)
A molecule of water weighs about 18 AMU ("atomic mass units"—a very tiny unit of weight!), an atom of Na weighs about 23 AMU, and Cl about 35 AMU. Question: take a volume of water and replace some of the molecules with Na's and Cl's; is the result heavier or lighter? (Prediction #4)


|
More:

Density
If you measure out equal volumes (at the same temperature) of fresh and salt water, which will weigh more? Why? This page will help you learn and understand the answers, and see how they affect phenomena in the estuary. A hands-on activity will give practice at observing and manipulating real systems showing these properties.
Put some water in a cup. How much water is it? There are several ways to answer this question, with slightly different results:
- Count how many molecules are present—huge number, very tiny, nearly impossible job!
- Measure the volume with a measuring cup, graduated cylinder, pipette, or ruler.
- Weigh the water on a scale (don't forget to subtract the weight of the container!)
- Now imagine a liter (exact volume) of fresh water and an exact liter of ocean water—do they weigh the same? If not, which weighs more? Why?
Dissolving salts in water results in atoms of Na and Cl filling the spaces between H2O molecules, adding their weight to the volume of water (without pushing the water out of the way very much). Thus the volume increases in weight—higher density, and more buoyancy.


|
More:

Buoyancy
Why do things float? Water pushes back when squeezed, and this pressure pushes on things that are immersed. When an object is put in a liquid, it displaces some volume of liquid previously there. That volume of liquid had been held in place by the pressure of the liquid around it, and that surrounding liquid still pushes with the same pressure as before, but now on the object displacing the liquid. The overall total of pressure is exactly the amount of force needed to hold up the missing (displaced) water; thus an object will sink into the water until it displaces a volume of water whose weight is equal to the object—at that point, the pressure on the object is just right to hold up the object's weight.
If the object has a weight greater than an equal volume of water (i.e. it's density is greater than the density of water) the water's force on it won't be enough to support it, and the object will sink. If the object's density is less than water's, it will float, part submerged (just enough to displace water equal to its weight); the rest of the object will remain above the surface—floating.


|
More:

So What is this Salt Wedge, anyway?
In estuaries, salt water meets fresh water. Let's make a miniature estuary and
study what happens!
Our study will be scientific—we'll make and record careful observations, try to explain what we see, use our explanations to predict what else might happen, then test our predictions. TEACHERS: practice this before having your students try—it takes patience to get really good results, but it's worth it! |
Materials
We'll need:
- salt (NaCl)—the cheapest kind often has the best crystals!
- water
- food coloring
- 2 measuring cups, or 2 clear plastic glasses
- PENCIL and PAPER!! (recording is important!!)
- optional magnifying glass or microscope
- paper towels in case of a spill
Procedure
- fill one glass or measuring cup about 2/3 way with water
- put NaCl in the other glass or cup, about 1/8 full or so
- take a pinch of the salt, look at it closely (microscope helps!)
- record your observations (sketch and/or verbal description)
- add water to the salt, about 1/4 glass, stir/slosh till dissolved—it's OK if some salt remains.
- Describe what happens
- If you have a microscope (40x is good), put a drop of water on some salt grains and watch patiently under the scope—describe!
- add some food coloring to make the very salty water dark
- very gently pour the salty water into the fresh water (it helps to tilt the fresh water glass to slow the pouring)
- observe and record the results!
We've got some explaining to do!
At least 3 good questions should come from your observations:
- Why are the NaCl crystals shaped like cubes?
- How did the salt disappear when water was added?
- Why does the salty water go to the bottom?
|




|
When ocean water comes into an estuary with the tide, it tends to settle to the bottom—just like our model. As it pushes its way in, it usually gets a wedge shape—hence the title of this Web Site!


More:

The Molecules of Photosynthesis & Respiration
|
Water H2O Carbon dioxide CO2
Photosynthesis
Plants, algae, and other living things containing chlorophyll can capture the energy of Sunlight and use it to tear apart and rearrange these two molecules (water, H2O + carbon dioxide CO2), in a process called "photosynthesis" (literally means "making with light").
After rearrangement, two new molecules are formed (using the very same atoms present originally: 3 O's (oxygen), one C (carbon), and 2 H's (hydrogen). One of the new molecules contains carbon, and H and O in the proportions of 2 H's to every O (like water, or "hydrate"), so it's called "carbohydrate". Examples are various types of sugar, starch, cellulose (in wood), and (shown below) formaldehyde, the simplest example.
Here it is as a chemical equation: H2O + CO2 + light energy -> CH2O + O2 (with the energy stored in the sugar)
Carbohydrate CH2O Diatomic oxygen O2
Some of the energy captured from the Sun is stored in the carbohydrate, making it a type of fuel. Like other fuels e.g. methane, natural gas in a kitchen stove, the energy can be released again by burning. In living things, this burning process is called respiration.
Respiration
Living things need energy to live (and all the processes associated with living: growing, digesting, moving,...). Where does this energy come from? It's stored in FOOD—a type of fuel, adsorbed by eating and digestion. How is the energy gotten out of the food/fuel? By burning it. Oxygen needed is obtained by breathing (air for land creatures, or from oxygen dissolved in water for water creatures). The oxygen and food combine in the cells of the body just as CH4 burns in a stove flame. The rearrangement of atoms is exactly the reverse of photosynthesis—CH2O + O2 -> H2O + CO2.
Practice these concepts in class with a hands-on activity, and observe them in action in mini-aquariums. |

H2O (water)

CO2 (carbon dioxide)

CH2O (Carbohydrate)

O2 (diatomic oxygen) |

 
|
More:

Salty Chalk Soda
This activity will study CO2 and carbonates (salts containing CO2), with an emphasis on how living things produce and use them.
Materials:
Chemicals:
- water H2O—distilled is best
- vinegar—acetic acid
- "sidewalk salt" of a specific kind—calcium chloride CaCl2
- baking soda—sodium bicarbonate NaHCO3
- chalk, seashells, limestone, coral,...various natural forms of calcium carbonate CaCO3
- table salt—sodium chloride NaCl (kosher salt dissolves best, but cheap salt has
best crystal shapes)
- seltzer—carbonic acid H2CO3 = H2O + CO2
Equipment:
- paper, pencils/pens, etc. for WRITING DOWN OBSERVATIONS
- bottle for water porting and waste liquid collection (Note: safe to dispose down drain)
- 3–4 medium (8 oz) clear plastic glasses
- plastic shot glass for each student
- paper towels
- masking tape for labels on shotglasses
- (optional, if available) digital camera and TV to attach it to
Students will do for themselves a "magic trick" (which the teacher can first perform in front of the class)—"making chalk appear from nowhere". We'll then study the chalk by testing it with acid (vinegar), and compare its reactions to other common substances, especially some made by living things in the ocean (e.g. chalk!).
Step-by-step procedure for the "experiment":
- Safety speech: keep away from eyes, faces, mouths, don't splash, no throwing around, especially at each other, NO TASTING!!
- Put bottle of water in view of class, commenting on "main ingredient of ocean".
- Distribute shotglasses among class; have paper towels handy; get students to prepare lab sheets for notes and drawings.
- Put some CaCl2 in one glass, some NaHCO3 in another.
- Add water, stir until dissolved, and discuss how its disappearance is a mystery we've solved earlier...
- Half of students in each group put a small piece of tape on their shotglass, as CaCl2 label.
- Give each student 1/2 shot of appropriate solution.
- Have pair of students (with different solutions) mix into UNLABELED shotglasses, observe what happens; let stand a while; write down procedure followed; everything observed; draw if inspired.
- While precipitate is settling, distribute chalk, rocks and shells; pick a shell (for TV/cameras display, if available); have all try to draw it.
- Ask if anyone knows what will happen if vinegar is added to baking soda? (Bet most do!). Demonstrate to class.
- Have them carefully decant leftover water in chalk glass into extra vessel, for later crystallization (hope to see NaCl cubes).
- Distribute small amounts of vinegar in now-empty shotglasses; have them test chalk, various rocks, shells, finally their precipitates.
- Last but not least—CLEANUP TIME!!
If still time left, take out bottle of seltzer; get class to describe everyday experiences (e.g. no bubbles visible till opening, opening after dropping, going flat...). Gasses dissolve, too! What gas is in soda? HINT: same as in our experiment—we made "salty chalk soda!"
If microscope available, set up, pass around artemia egg samples for examination, then put under microscope to show how much magnification the microscope provides; do the same for for NaCl crystals (use brand of salt with good crystals! cheap generic is usually fine).


|
![]()
![]()

![]()
![]()






 OR
OR  OR
OR  ?
?

![]()



























